Darya is a crypto enthusiast who strongly believes in the future of blockchain. Being a hospitality professional, she is interested in finding the ways blockchain can change different industries and bring our life to a different level.
As the draft of a form letter to UK AstraZeneca vaccine trial participants states, FDA “has come to the same conclusion as the other drug regulators including the MHRA” and approved further vaccine testing.

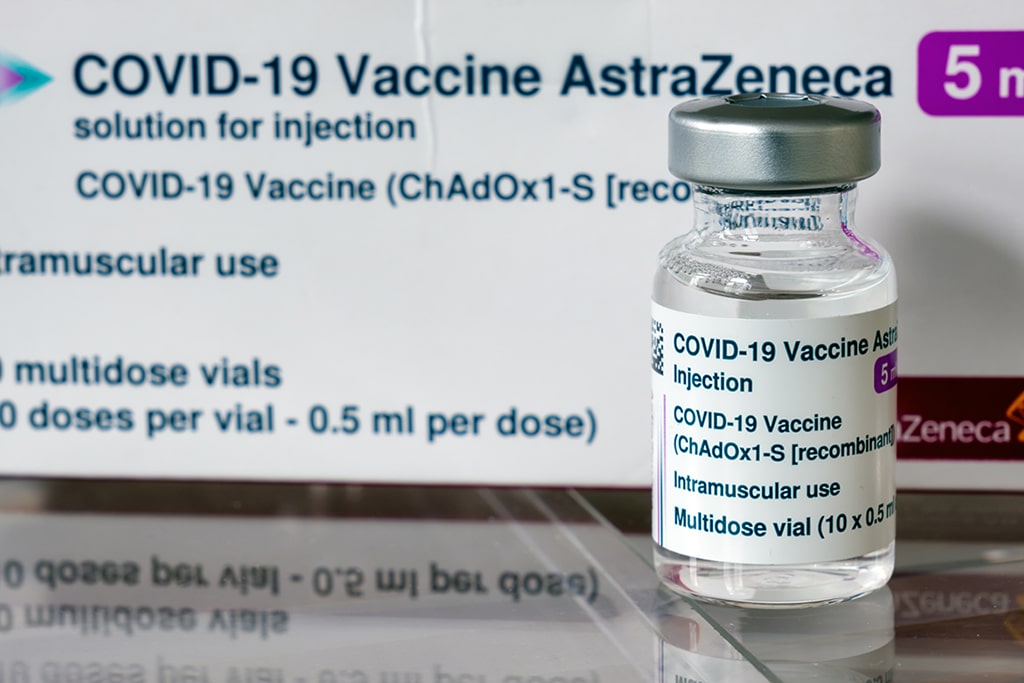
Some may have knocked AstraZeneca plc (NASDAQ: AZN) out of the reckoning, but the company seems to be on the way back to the coronavirus vaccine race. After more than a monthlong pause, AstraZeneca may resume the COVID-19 vaccine trial this week. The US Food and Drug Administration (FDA) has already completed its review of safety data and given a green light for further testing.
To develop the vaccine, AstraZeneca has been collaborating with Oxford University. The partners have shown significant progress in the development, reaching late-stage Phase 3 trials in the U.S. However, on September 8, AstraZeneca announced trial suspension following a serious suspected adverse reaction found in a participant. The British-Swedish pharma giant had to conduct a safety review and investigate whether the vaccine caused the illness.
A week later, after UK’s Medicines Health Regulatory Authority reviewed the drug, AstraZeneca joined the race for developing a COVID-19 vaccine again. The UK regulatory officials determined there was “insufficient evidence to say for certain” that the illness was or was not related to the vaccine. The trial was allowed to enroll participants in the UK again. Besides, the company received partial immunity from the European government. It secured backing from the European Union in a confidential agreement that offers the drugmaker a liability waiver. Under the deal, European Union countries would be paying $2.92 (2.5 euros) per dose for AstraZeneca’s vaccine.
At the beginning of October, AstraZeneca resumed clinical trials of its vaccine candidate in Japan. Brazil, India, and South Africa allowed AstraZeneca to resume its vaccine trials there as well. The company has also started talks with United States authorities to recommence the vaccine trial process. Notably, AstraZeneca was awarded $486 million by the United States government to supply 100k vaccine doses. According to anonymous sources, the US trial may resume this week.
As the draft of a form letter to UK vaccine trial participants states, FDA “has come to the same conclusion as the other drug regulators including the MHRA”. FDA has not commented on the news.
Due to the company’s progress in COVID-19 vaccine development, AZN stock has been in a solid bull rally this year. In the past twelve months, AZN shares have added 24.82%. In addition, they have jumped by 6.03% year to date through Wednesday. AstraZeneca’s current market cap is $136.906 billion.
Yesterday, AstraZeneca stock closed 0.48% down, at $52.19. Its 52-week high is $64.94, 52-week low is $36.15.
Disclaimer: Coinspeaker is committed to providing unbiased and transparent reporting. This article aims to deliver accurate and timely information but should not be taken as financial or investment advice. Since market conditions can change rapidly, we encourage you to verify information on your own and consult with a professional before making any decisions based on this content.

Darya is a crypto enthusiast who strongly believes in the future of blockchain. Being a hospitality professional, she is interested in finding the ways blockchain can change different industries and bring our life to a different level.