AZN Stock Up 1% in Pre-market, AstraZeneca Vaccine Found to Be 79% Effective in US with No Safety Issues
AstraZeneca anticipates the vaccine to continue being shipped to different countries to combat the coronavirus spread.

AstraZeneca anticipates the vaccine to continue being shipped to different countries to combat the coronavirus spread.

Notably, the World Health Organization has approved two different versions of the AstraZeneca vaccine that were found out to be approximately 63% effective.

As part of the massive $39 billion deal, AstraZeneca has agreed to pay a whopping 45% of the share price of Alexion. As per analysts, this could be a win-win situation for both companies.

On Thursday AstraZeneca said it would undertake a new global vaccine trial using the lower-dose regimen.
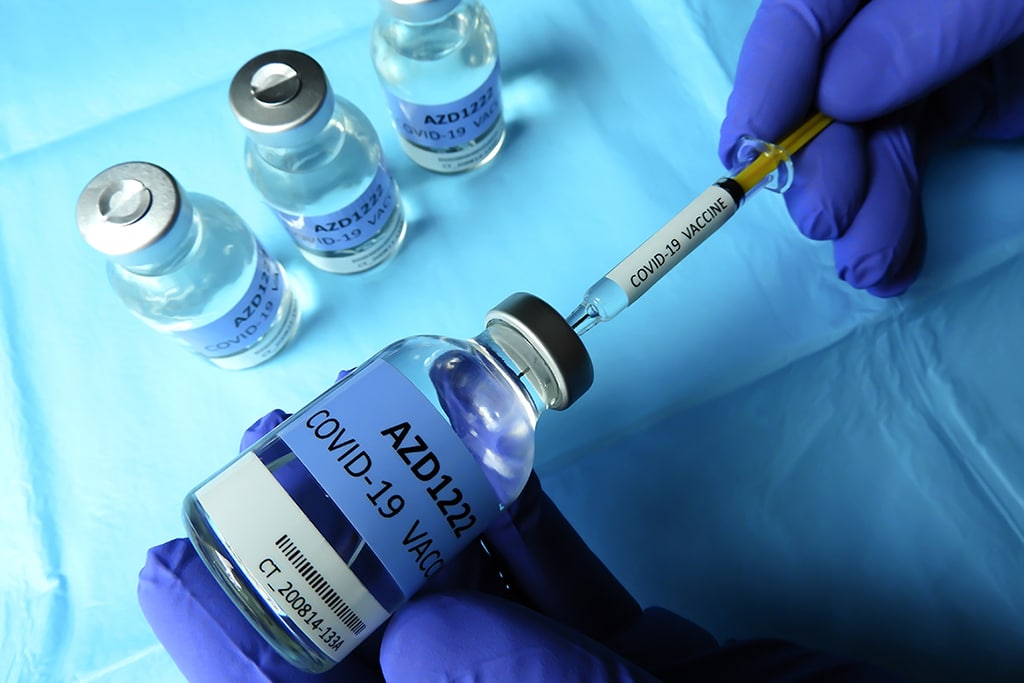
Currently, AstraZeneca vaccine trials are in the late-stages, involving about 23,000 participants. The participants span across the UK, U.S., South Africa, and Brazil.

The British health regulators will monitor clinical data in real-time while having an active dialogue with the drugmaker to accelerate the approval process.

As the draft of a form letter to UK AstraZeneca vaccine trial participants states, FDA “has come to the same conclusion as the other drug regulators including the MHRA” and approved further vaccine testing.

In partnership with U.K.’s Oxford University, AstraZeneca said that it will resume the phase three clinical trial for its COVID-19 vaccine contender AZD1222 since it got an approval from UK’s Medicines Health Regulatory Authority.

The Phase 3 trial started on August 31 and enrolled about 30,000 participants at 80 locations. Now it is unclear how long the clinical hold will last. But it is obvious that other pharma companies will have to review their safety measures as well.

With the company’s ongoing late-stage clinical trials and its impressive H1 performance results, AstraZeneca (AZN) stock jumped in the pre-market.

Data from AstraZeneca’s clinical trials of its COVID-19 vaccine candidate AZD1222 shows promising results but AZN share price has refused to be positively impacted.
AstraZeneca’s COVID-19 vaccine clinical trials gave possible results. Hopes to see the speedy release of the vaccine boost AZN stock.

Within AstraZeneca’s agreement with Europe’s Inclusive Vaccines Alliance (IVA), France, Germany, Italy, and the Netherlands will accelerate the supply of the vaccine. AZN stock is up in the pre-market today.

If combined, AstraZeneca and Gilead Sciences would have a market capitalization of about $237 billion, exceeding Merck & Co and Pfizer worth $207 billion and $200 billion respectively.

AstraZeneca plans to deliver 1 billion doses of a COVID-19 vaccine to developing countries, 400 million doses can become available this year. Moreover, 400 million doses will be sent to the U.S. and the United Kingdom. AZN stock is up in the pre-market.